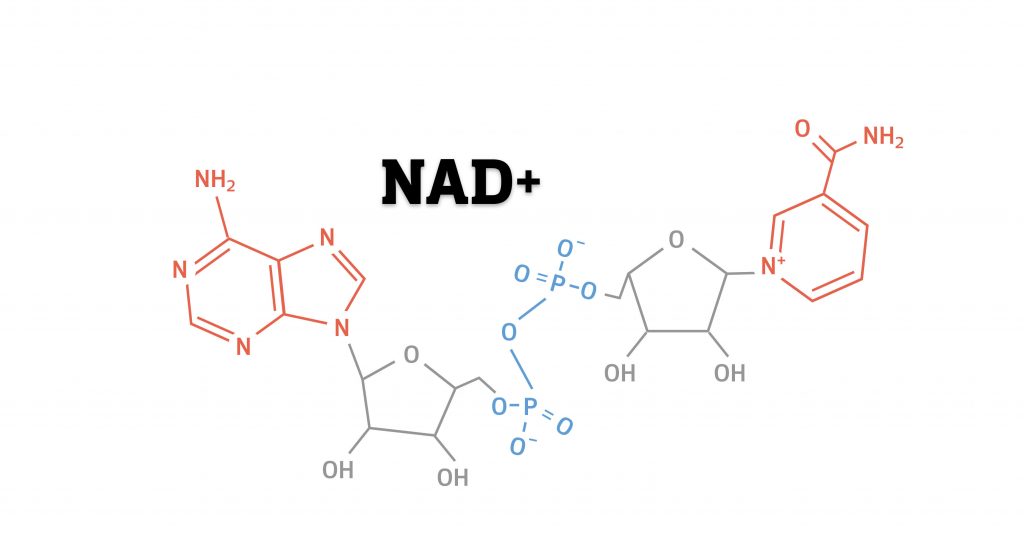
At the core of the trillions of cells that make up the human body, each choreographing countless processes, sits NAD+ (nicotinamide adenine dinucleotide). This essential cellular molecule is made of the same building blocks as DNA – nucleotides. While DNA is made up of billions of nucleotides, NAD+ is rather small – composed of just two. But size doesn’t matter here – NAD+ serves so many vital functions in the cell that without it we would not survive.
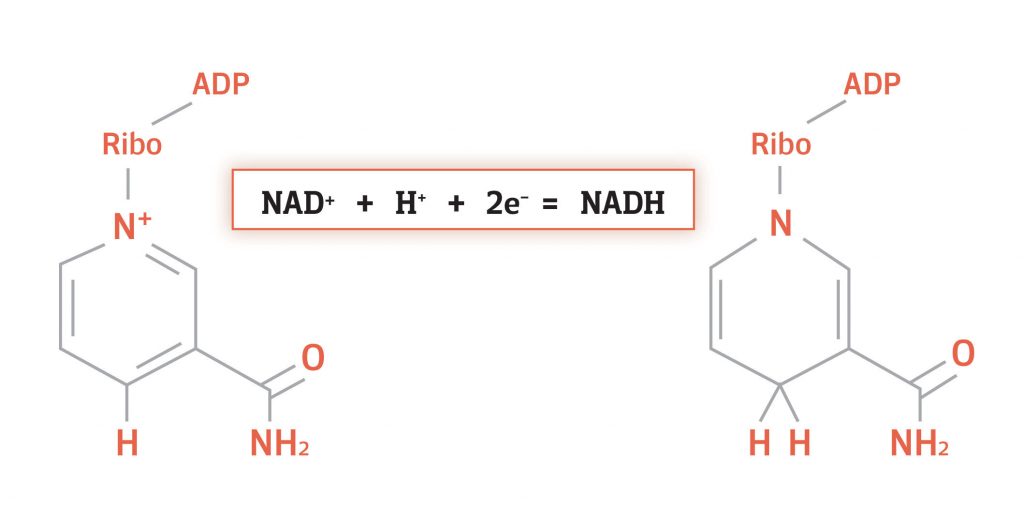
NADH is very similar to NAD+, the only difference is a hydride. What’s a hydride? A hydride is a hydrogen atom with an extra electron. That is, a neutrally charged hydrogen atom holding on to a negatively charged electron. This gives hydride an overall negative charge:
H + e- = H-
Here, the hydrogen atom is denoted by an H, the electron by an e-, and the hydride by an H-. Since NAD+ is positively charged, adding a hydride to an NAD+ molecule results in a neutrally charged NADH molecule:
NAD+ + H- = NADH
To summarize, the difference between NAD+ and NADH is a hydride. NADH is bound to a hydride and NAD+ is not. Importantly, the hydride contains electrons and this is why NAD+ is considered an electron carrier.
NAD+ the Electron Carrier
NAD+ is an electron carrier because it carries the electrons of a hydride from one region of a cell to another. The structure of NAD+ provides a stable shuttle for the movement of electrons across the reactive landscape that is the internal environment of a cell. Without molecules like NAD+, electrons could hardly make it to their proper destination. This process of moving electrons around makes NAD+ and NADH oxidation-reduction, or “redox” molecules.
Oxidation and Reduction
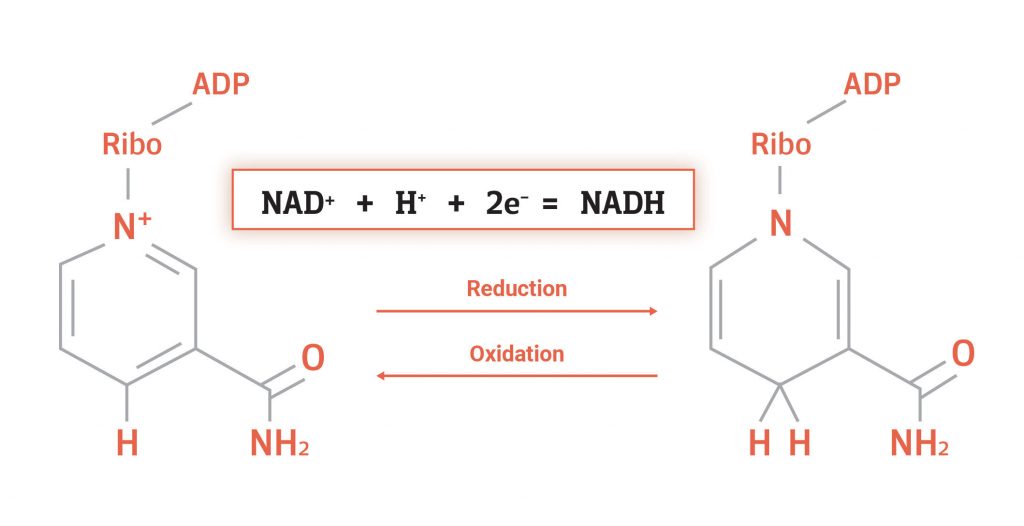
Oxidation and reduction are terms to describe when molecules gain or lose electrons. Reduction is when a molecule gains electrons, such as when NAD+ gains the electrons from a hydride to become NADH. In this case, we say that NAD+ has been reduced to NADH.
Oxidation is when a molecule loses electrons, such as when NADH loses its hydride to become NAD+. In this example, we say that NADH is oxidized to NAD+. This process is by no means permanent and can occur repeatedly in the same molecule. This means that NAD+ can continuously gain and lose electrons, emphasizing its electron carrier status.
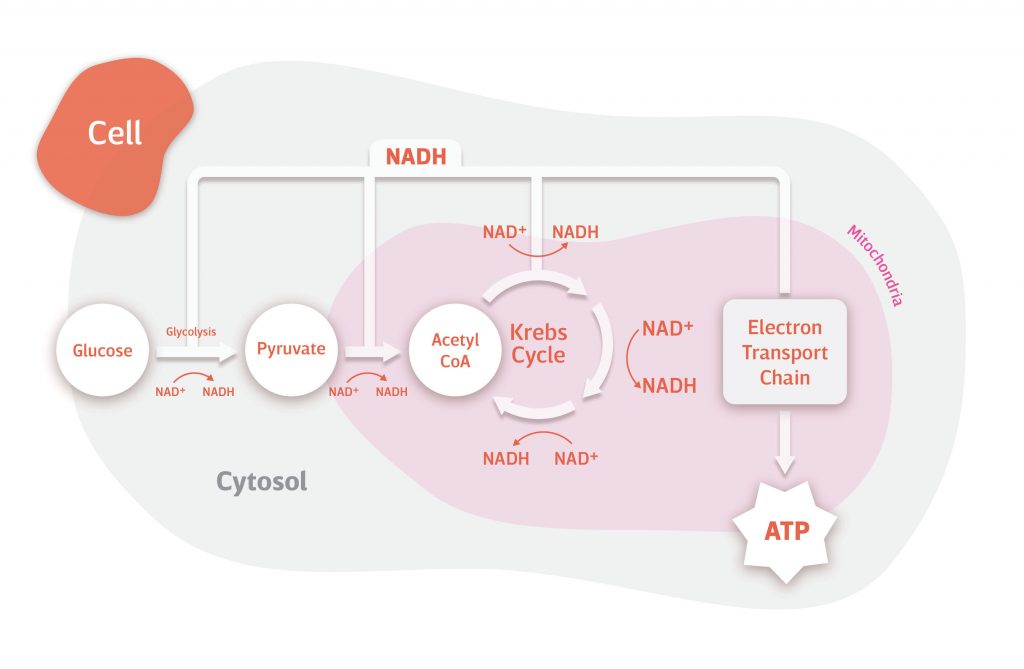
NAD+ and NADH in Action
How do NAD+ and NADH contribute to providing us with the energy we need to survive? Without the electron carrying capacity of NAD+, our cells wouldn’t be able to metabolize food into adenosine triphosphate (ATP), the energy used by our cells to keep us alive.
Food is packed with fuel – in the form of macromolecules like carbohydrates, proteins, fats – just waiting to be extracted and transformed into ATP. However, in most cases, ATP isn’t just prepackaged and ready to go – our cells have to make it from scratch. NAD+ is what makes this possible.
The importance of NAD+ in transforming macromolecules into ATP can be illustrated by how it harnesses the energy from the breakdown of carbohydrates. Carbohydrates are broken down by our digestive system into sugar molecules, most commonly glucose and sometimes fructose.
Glucose travels through our bloodstream until it permeates our cells, usually in response to insulin. Once in the cell, it is broken down into smaller and smaller molecules through a series of reactions. During many of these reactions, the chemical bonds that hold glucose together are broken. It’s the energy trapped within these bonds that are harnessed by NAD+ to produce ATP.
Of the nineteen reactions involved in the full breakdown of glucose (glycolysis → conversion of pyruvate to acetyl-CoA → Krebs/citric acid/TCA cycle), NAD+ is involved in five. During these five reactions, an electron is ejected from the molecule being broken down and captured by NAD+. In this process, NAD+ is reduced to NADH, harnessing the energy freed from the broken chemical bond.
NADH carries the electrons gained from the breakdown of glucose and donates them to the chain of enzymes in mitochondria that are involved in producing ATP (electron transport chain). This oxidizes NADH back to NAD+. Ultimately, the energy from the electrons donated by NADH is exploited by mitochondria to produce ATP (oxidative phosphorylation).
Summary
To summarize some of the differences between NAD+ and NADH:
- NADH is bound to a hydride and NAD+ is not bound to a hydride.
- NADH is actively carrying electrons and NAD+ is not carrying electrons.
- NADH is the reduced form of NAD+.
- NAD+ is the oxidized form of NADH.
Overall, in the context of metabolizing food for energy, NAD+ and NADH are the same molecule, NADH is simply the electron-carrying form of NAD+.